Acid Rain: Unveiling the Invisible Catastrophe
Acid rain is a less visible but profoundly destructive environmental issue that quietly wreaks havoc on our ecosystems, infrastructure, and health. Its effects might not be as immediately apparent as those of a hurricane or wildfire, but its long-term impact is equally devastating. In this comprehensive blog, we’ll explore the origins, mechanisms, consequences, and solutions related to acid rain, shedding light on this often-overlooked threat.
What is Acid Rain?
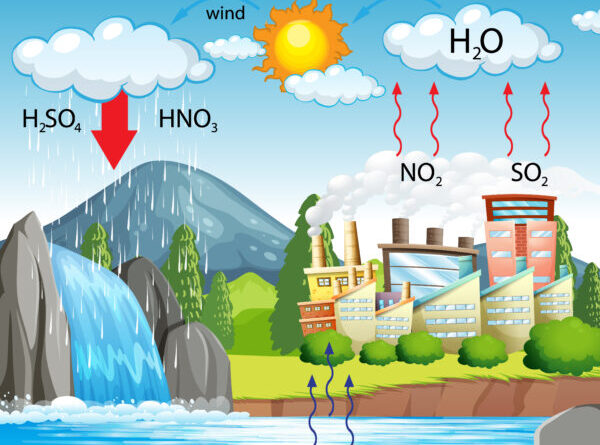
Acid rain is a type of precipitation that has elevated levels of hydrogen ions (low pH), making it acidic. This phenomenon can manifest as rain, snow, fog, hail, or even dust. The primary culprits behind acid rain are sulfur dioxide (SO2) and nitrogen oxides (NOx), which are released into the atmosphere through both natural and anthropogenic activities. These pollutants undergo complex chemical transformations in the atmosphere, resulting in the formation of sulfuric and nitric acids, which then mix with cloud moisture and fall as acid precipitation.
How Does Acid Rain Form?
The process of acid rain formation unfolds through a series of distinct stages:
1. Emission of Pollutants:
- Anthropogenic Sources: The burning of fossil fuels in power plants, industrial facilities, and vehicles is the primary source of SO2 and NOx emissions. Coal-burning power plants are particularly notorious for emitting large quantities of sulfur dioxide.
- Natural Sources: Volcanic eruptions, forest fires, and lightning strikes also contribute to the atmospheric load of these pollutants, but to a lesser extent compared to human activities.
2. Atmospheric Transformation:
- Once released, SO2 and NOx can travel long distances in the atmosphere. During their journey, they react with water vapor, oxygen, and other chemicals to form sulfuric and nitric acids.
3. Deposition:
- These acids then combine with precipitation and fall to the Earth as acid rain. The acidic particles can also be deposited in a dry form, settling on surfaces and later mixing with water to form acid rain.
The Devastating Effects of Acid Rain:
Acid rain affects various aspects of the environment and human society. Here’s a detailed look at its multifaceted impacts:
4. Environmental Impact:
- Aquatic Ecosystems: Acid rain significantly lowers the pH of water bodies, making them too acidic for many aquatic organisms to survive. Fish eggs and larvae are particularly sensitive, leading to reduced fish populations. Acidic waters also dissolve aluminum from soil, which further contaminates water bodies and is toxic to fish.
- Terrestrial Ecosystems: Forests and soils suffer immensely from acid rain. The acidic deposition leaches essential nutrients like calcium and magnesium from the soil, weakening trees and other vegetation. This nutrient depletion makes plants more susceptible to diseases, pests, and harsh weather.
5. Biodiversity Loss:
- The decline in fish populations affects the entire food web, impacting species that rely on fish for sustenance, such as birds and mammals. Terrestrial plants, weakened by nutrient loss, fail to support the diverse insect and animal life that depends on them, leading to a decline in biodiversity.
6. Human Health:
- While acid rain does not pose direct health risks, the pollutants that cause it (SO2 and NOx) are harmful. These pollutants can cause or exacerbate respiratory diseases like asthma and bronchitis, particularly in vulnerable populations such as children and the elderly.
7. Damage to Infrastructure:
- Acid rain accelerates the decay of buildings, bridges, and other structures, especially those made of limestone and marble. Historic monuments and cultural heritage sites are particularly vulnerable. The acid reacts with the calcium carbonate in these materials, causing them to erode and deteriorate over time. This not only leads to increased maintenance costs but also threatens the preservation of our cultural history.
Global Efforts to Combat Acid Rain:
Given its wide-reaching effects, combating acid rain requires a concerted global effort. Here are some strategies and measures that have been implemented:
8. Regulatory Measures:
- Emission Reductions: Governments worldwide have introduced regulations to limit SO2 and NOx emissions. For example, the Clean Air Act Amendments of 1990 in the United States established the Acid Rain Program, which set a cap on SO2 emissions from power plants.
- Emission Trading Systems: Cap-and-trade programs allow industries to buy and sell emission allowances, providing economic incentives to reduce emissions. This market-based approach has been successful in significantly lowering sulfur dioxide levels in the U.S.
9. Transition to Clean Energy:
- Shifting from fossil fuels to renewable energy sources is crucial. Solar, wind, and hydroelectric power generate electricity without emitting SO2 and NOx. Governments and private sectors are increasingly investing in these technologies to reduce reliance on coal and other polluting energy sources.
10. Technological Innovations:
- Scrubbers: These devices, installed in smokestacks of power plants and industrial facilities, remove sulfur dioxide from exhaust gases before they are released into the atmosphere.
- Catalytic Converters: Vehicles equipped with catalytic converters can significantly reduce NOx emissions. These devices convert nitrogen oxides into nitrogen and oxygen, which are harmless.
11. International Cooperation:
- Acid rain is a transboundary issue, as pollutants can travel long distances across borders. International treaties and agreements, such as the Gothenburg Protocol under the Convention on Long-Range Transboundary Air Pollution (CLRTAP), aim to reduce air pollution on a regional scale by setting emission reduction targets for participating countries.
12. Public Awareness and Education:
- Raising awareness about the causes and effects of acid rain can drive behavioral changes. Educating the public about energy conservation, supporting clean energy initiatives, and advocating for stricter environmental regulations are vital components of this effort.
Personal Actions to Reduce Acid Rain:
Individuals can also play a crucial role in reducing acid rain. Here are some actions you can take:
13. Energy Conservation:
- Reducing energy consumption at home and work can lower the demand for electricity, thereby reducing the amount of fossil fuels burned. Simple actions like turning off lights, using energy-efficient appliances, and insulating homes can make a big difference.
14. Supporting Clean Energy:
- Choosing renewable energy options where available, such as opting for a green energy plan from your utility provider, can contribute to a decrease in acid rain-causing emissions.
15. Sustainable Transportation:
- Reducing car use by walking, biking, carpooling, or using public transportation can lower NOx emissions. Additionally, driving fuel-efficient or electric vehicles further reduces your personal contribution to air pollution.
16. Advocacy and Participation:
- Supporting policies and politicians that prioritize environmental protection and advocating for stronger environmental regulations can help drive systemic change.
Conclusion:
Acid rain, though less visible than other environmental disasters, poses a serious threat to our ecosystems, human health, and cultural heritage. By understanding its causes and effects, and through coordinated efforts at the individual, national, and international levels, we can mitigate its impact and protect our planet for future generations. The battle against acid rain exemplifies the broader fight against pollution and environmental degradation—a fight that requires vigilance, innovation, and a shared commitment to sustainability.
FAQs:
1. What is acid rain?
Acid rain refers to any form of precipitation (rain, snow, fog, hail, or dust) that contains elevated levels of sulfuric and nitric acids. This occurs when sulfur dioxide (SO2) and nitrogen oxides (NOx) are emitted into the atmosphere and chemically interact with water vapor.
2. What are the primary causes of acid rain?
The main causes of acid rain include human activities such as the combustion of fossil fuels (coal, oil, and natural gas) in power plants, industrial processes, and motor vehicles. Natural events like volcanic eruptions and wildfires also contribute to a lesser extent.
3. How does acid rain develop?
Acid rain forms through a series of chemical reactions. SO2 and NOx released into the atmosphere react with water vapor, oxygen, and other chemicals to produce sulfuric and nitric acids. These acids then combine with precipitation, falling to the Earth as acid rain.
4. What impact does acid rain have on the environment?
Acid rain significantly impacts the environment:
- Aquatic ecosystems: It lowers the pH of water bodies, making them more acidic and harmful to fish and other aquatic organisms.
- Forests: It depletes essential nutrients from the soil, weakening trees and plants and making them more susceptible to disease and harsh weather.
- Biodiversity: It reduces populations of sensitive species, leading to disrupted ecosystems and loss of biodiversity.
5. How does acid rain affect human health?
While acid rain does not directly harm human health, the pollutants that cause it (SO2 and NOx) can lead to respiratory conditions such as asthma and bronchitis. These pollutants also contribute to the formation of fine particulate matter, which is hazardous when inhaled.
6. Can acid rain damage buildings and monuments?
Yes, acid rain can accelerate the deterioration of buildings, monuments, and statues, particularly those made from materials like limestone and marble. The acids react with calcium carbonate in these materials, leading to erosion and structural damage.
7. How is acid rain measured?
Acid rain is measured by collecting samples of precipitation and analyzing their pH levels. A pH value below 5.6 indicates acid rain, as natural, unpolluted rainwater typically has a slightly acidic pH of around 5.6 due to carbonic acid formed from carbon dioxide and water.
8. Which regions are most affected by acid rain?
Regions with high levels of industrial activity and fossil fuel combustion, such as parts of North America, Europe, and Asia, are most affected by acid rain. However, air pollution can travel long distances, meaning even remote areas can be impacted.